
Pharma Service
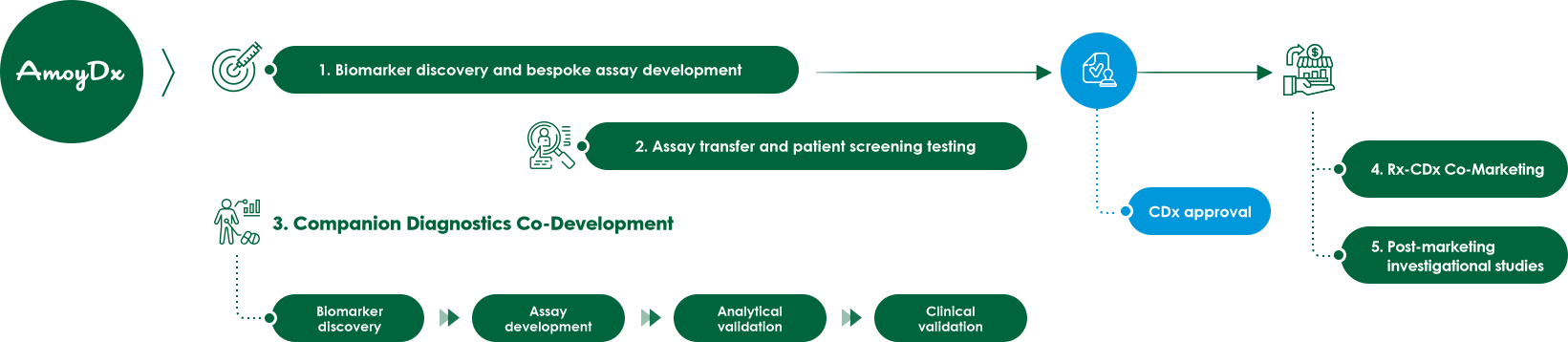
As a pioneer in companion diagnostics (CDx), AmoyDx highly values our partnerships with pharmaceutical companies, and we are committed to continuously developing innovative diagnostics approaching precision medicine, delivering tailored treatments to maximize clinical benefits.From early development to regulatory approval, we offer comprehensive solutions to accelerate drug development, including drug related biomarker discovery, bespoke assay development, global assay transfer, CTA service, companion diagnostics kits development and post-marketing investigational studies.
Laboratory Network
US CLIA and CAP
Accredited Labs
EU CAP& ISO15189
Accredited Labs
Asia CAP & ISO15189
Accredited Labs
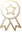
We’ve built a global network of CAP accredited labs and partners to provide harmonized laboratory testing service across the world. So far, our laboratory testing service has supported 400+ clinical trials around the globe, including China, APAC, EMEA and US, and a wide range of technologies has been implemented in laboratory testing, including PCR, NGS, IHC/mIHC/Digital pathology, FISH, Sanger Sequencing, and MS.
CDx Development

FDA
Food and Drug Administration

EMA
European Medicines Agency

NMPA
National Medical Products Administration

MFDS
Ministry of Food and Drug Safety

PMDA
Pharmaceuticals and Medical Devices Agency

AmoyDx has extensive experience of CDx/IVD development around the globe. So far we have developed and commercially launched 70+CDx/IVD products globally, with CDx registration and regulatory submission experience in China, APAC, EMEA and US , the technologies of these CDx/IVD products are based on PCR, NGS, IHC, FISH and Sanger Sequencing.
Global
Laboratory Network
We’ve built a global network of CAP accredited labs and partners to provide harmonized laboratory testing service across the world. So far, our laboratory testing service has supported 400+ clinical trials around the globe, including China, APAC, EMEA and US, and a wide range of technologies has been implemented in laboratory testing, including PCR, NGS, IHC/mIHC/Digital pathology, FISH, Sanger Sequencing, and MS.
Global
CDx Development
AmoyDx has extensive experience of CDx/IVD development around the globe. So far we have developed and commercially launched 70+CDx/IVD products globally, with CDx registration and regulatory submission experience in China, APAC, EMEA and US , the technologies of these CDx/IVD products are based on PCR, NGS, IHC, FISH and Sanger Sequencing.
Press Release

Global
Presence
More
Get in Touch