- All
- 2026
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015

AmoyDx® FGFR2 Break-apart FISH Probe Kit Approved as Companion Diagnostic for Eisai’s Tasurgratinib in Japan
AmoyDx® FGFR2 Break-apart FISH Probe Kit has received approval in Japan as a companion diagnostic (CDx) for Eisai’s groundbreaking targeted therapy, Tasurgratinib.

AmoyDx® Pan Lung Cancer PCR Panel Approved in Japan as a Companion Diagnostic for Haiyitan® tablets (gumarontinib)
The Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the AmoyDx® Pan Lung Cancer PCR Panel (the “AmoyDx PLC Panel”) as a companion diagnostic for Haiyitan® (gumarontinib), a product of Haihe Biopharma K.K. Haiyitan® in 50 mg tablet form, was approved by MHLW in June 2024 for patients with unresectable advanced or recurrent non-small cell lung cancer (NSCLC) with MET exon 14 (METex14) skipping mutations.

AmoyDx and Servier Enter into a Collaboration to Develop an IDH1/2 Companion Diagnostic Test for Diffuse Glioma in China
AmoyDx and Servier entered into a strategic partnership to develop a companion diagnostic test (CDx) in China that detects Isocitrate Dehydrogenase (IDH) 1&2 gene mutations.

AmoyDx Wins National Manufacturing Single Champion Enterprise Title!
AmoyDx has proudly made the list of manufacturing champions by the Ministry of Industry and Information Technology.

AmoyDx Collaborates with Boehringer Ingelheim to Develop Companion Diagnostics for Lung Cancer Patients in China
AmoyDx announced a collaboration with Boehringer Ingelheim to develop a Companion Diagnostic (CDx) kit for non-small cell lung cancer (NSCLC) patients in China.

AmoyDx® Pan Lung Cancer PCR Panel Approved in Japan as a Companion Diagnostic for TABRECTA (Capmatinib)
AmoyDx® Pan Lung Cancer PCR Panel has been approved by the Japanese Ministry of Health, Labour and Welfare (MHLW) as a companion diagnostic for TABRECTA™ (capmatinib)
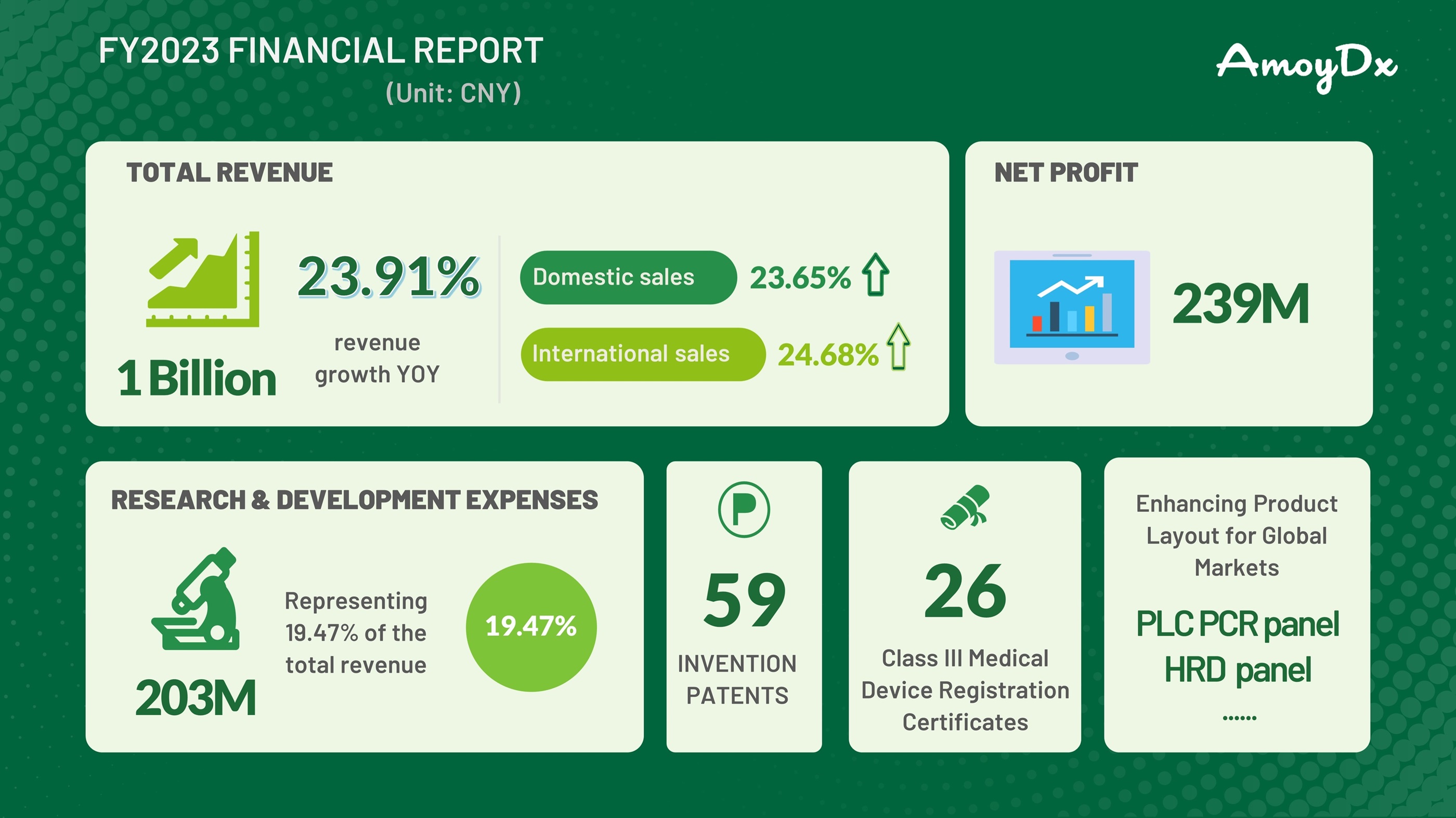
Highlights of AmoyDx FY2023 Financial Report
Amoy Diagnostics Co., Ltd (AmoyDx SZSE: 300685) reported its financial results for the fiscal year 2023.

Revolutionizing Cancer Care in Vietnam: AmoyDx, BCE Vietnam and VSPC Sign MOU to Launch Overseas Molecular Pathology Training Initiative
AmoyDx and BCE Vietnam had signed a Memorandum of Understanding (MOU) with the Vietnam Society of Pathology and Cytology (VSPC) to launch an independent educational initiative committed to Overseas Molecular Pathology Training.

AmoyDx® PD-L1 (E1L3N) assay gained the priority recommendation from Chinese expert consensus
The AmoyDx®PD-L1 (E1L3N) assay was prioritized as companion diagnostics for Pembrolizumab.

AmoyDx has successfully held the 2023 Distributors Annual Meeting at the headquarter in Xiamen, China, on January 22-23, 2024
AmoyDx has successfully held the 2023 Distributors Annual Meeting at the headquarter in Xiamen, China, on January 22-23, 2024

LC-SCRUM's 10-Year Milestone: Amoy Diagnostics Earns International Recognition for Excellence
Dr. Limou Zheng, Founder of Amoy Diagnostics, and Mr. Frank Zheng, Chief Operating Officer, were invited to give a keynote speech at the 10th-anniversary conference of the Asian Lung Cancer Genome Screening Project (LC-SCRUM)

Highlights of China International Import Expo (CIIE) 2023 - Amoy Diagnostics Announced Multiple Collaborations with AstraZeneca and Pfizer
AmoyDx announced multiple collaborations with AstraZeneca and Pfizer during the 2023 China International Import Expo (CIIE), held on November 5-10 in Shanghai China. These partnerships aim to jointly promote the development of precision diagnostics

AmoyDx® Microsatellite Instability (MSI) Detection Kit Received NMPA Approval for Pan-Tumor Immunotherapy Companion Diagnostics
National Medical Products Administration (NMPA) granted approval to AmoyDx® Microsatellite Instability (MSI) Detection Kit

AmoyDx Collaborates with AstraZeneca to Develop a HER2 Companion Diagnostic for Lung Cancer in China
We are thrilled to announce that AmoyDx reaches a new collaboration agreement with AstraZeneca. Under this agreement, the AmoyDx® Essential NGS panel will be used as a companion diagnostic of ENHERTU® to identify HER2 (ERBB2) mutations in patients with no

AmoyDx Signed a Collaboration Agreement with AstraZeneca for HRD Companion Diagnostic
Amoy Diagnostics Co., Ltd (AmoyDx), a China-based innovative molecular diagnostics company (SZSE: 300685), announced that it has entered into a Companion Diagnostic Collaboration Agreement with AstraZeneca (LSE/STO/Nasdaq: AZN), a global biopharmaceutical

AmoyDx® Pan Lung Cancer PCR Panel Approved in Japan as a Companion Diagnostic for selpercatinib RET fusion positive NSCLC
The Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the AmoyDx® Pan Lung Cancer PCR Panel (the “AmoyDx PLC Panel”) as a companion diagnostic for RET fusion-positive non-small cell lung cancer (“NSCLC”), for selpercatinib capsules 40 mg

Career Opportunities - Business Manager (EMEA)
You are looking for opportunities to bring to use your knowledge of and experience in oncology molecular diagnostics in qPCR and Next Generation Sequencing application? You are passionate in building meaningful relationship with both internal and external

Career Opportunities - The Field Application Scientist
The Field Application Scientist is responsible for system usage training at customer sites while providing PCR/NGS experimental design, data analysis, logistical, and troubleshooting support for LATAM.
