The AmoyDx® MSI PCR Kit is a qualitative real-time PCR assay for detecting microsatellite instability (MSI) in formalin-fixed, paraffin embedded (FFPE) colorectal cancer tissue samples. Intended for research use only (RUO), the kit requires operation by trained professionals in a controlled laboratory setting. It is compatible with the LightCycler480 II and SLAN-96S platforms for assay performance and data acquisition. Results shall be analyzed using ARAS, a dedicated software tool optimized for AmoyDx® MSI PCR Kit outputs.
The AmoyDx® MSI PCR Kit employs advanced melting curve analysis integrated with real-time PCR, leveraging specific primers and multi channel fluorescent probes (FAM, VIC, CY5) to assess instability at six key microsatellite loci (ACVR2A, PRR5-ARHGAP8, EIF4E3, IFT140, RBM14-RBM4, UBAC2). This enables precise evaluation of microsatellite instability (MSI) status in formalin-fixed, paraffin-embedded (FFPE) colorectal cancer tissue samples. Based on the number of unstable loci detected, the kit classifies samples into two categories: microsatellite instability-high (MSI-H) or non-high microsatellite instability (MSI-L/MSS). Furthermore, the kit's PCR amplification system incorporates uracil-N-glycosylase (UNG) enzyme, which selectively hydrolyzes uracil-glycosidic bonds in dU-containing amplicons, thereby minimizing false-positive results due to carryover contamination from prior PCR products.
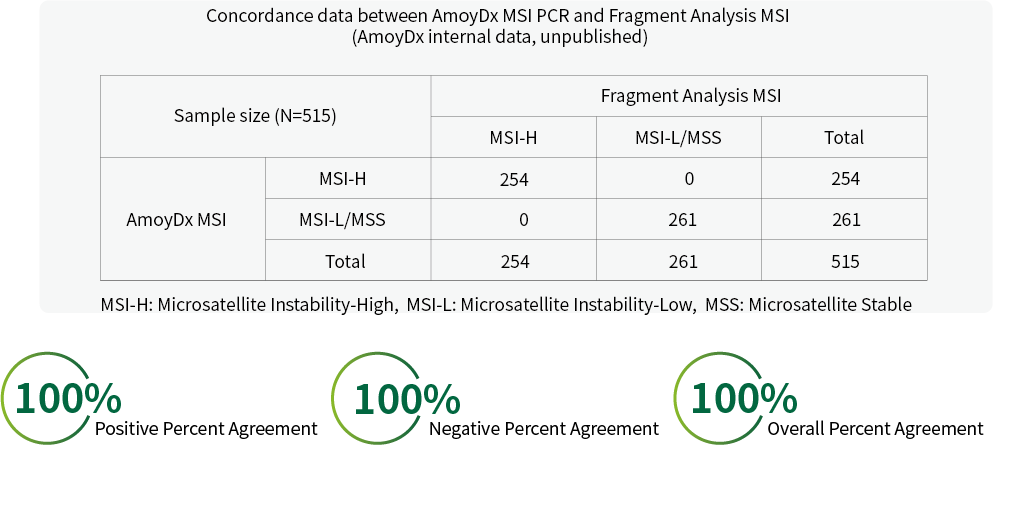
High Concordance Rate with Fragment Analysis MSI

Specifications
Tests/kit
24 (Pre-loaded)
Instrument
LighCycler480 II, SLAN-96S
Inquiry